Kauna-unahang COVID-19 self-testing kit inaprubahan ng US-FDA para sa ‘home use’
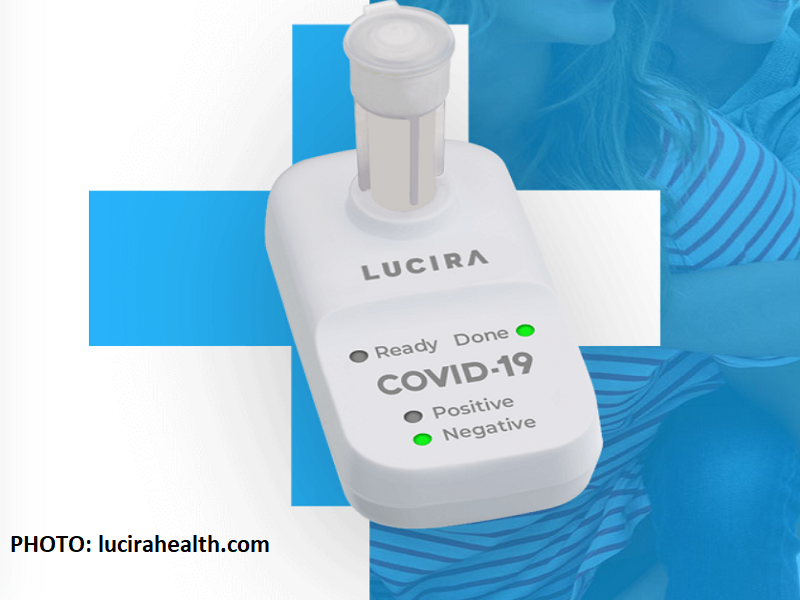 Inaprubahan na ng U.S. Food and Drug Administration ang kauna-unahang COVID-19 self-testing kit na pwede sa home use.
Inaprubahan na ng U.S. Food and Drug Administration ang kauna-unahang COVID-19 self-testing kit na pwede sa home use.
Ang single-use test ay kayang makapaglabas ng resulta sa loob ng 30-minuto lamang.
Gawa ito ng kumpanyang Lucira Health na napagkalooban na ng emergency use authorization para sa home use.
Ginagamitan din ang test ng self-collected nasal swab samples para sa mga indibidwal na edad 14 at pataas na suspected COVID-19 patient.
Ayon kay FDA Commissioner Stephen Hahn, ito ang maituturing na unang self-administered COVID-19 test kit.
Pwede ring gamitin ang kit sa mga pagamutan.
Disclaimer: The comments uploaded on this site do not necessarily represent or reflect the views of management and owner of Cebudailynews. We reserve the right to exclude comments that we deem to be inconsistent with our editorial standards.

